CTEP Branches and Offices
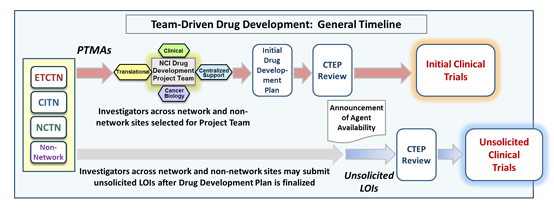
NCI Drug Development Project Teams
The NCI Experimental Therapeutics (NExT) Program approves certain high-priority agents for development by NCI Drug Development Project Teams, in which NCI grantees collaborate with the NCI in multi-institutional, multi-disciplinary teams to design NCI’s initial drug development plan for these therapies.
Several times a year, CTEP will solicit and review Project Team Member Applications (PTMAs) for an agent (or multiple agents) in order to determine the makeup of the agent Project Team. The teams determine which clinical trials will be conducted across the NCI CTEP clinical trials network sites, and how best to approach critical translational studies. The subsequent NCI drug development work will be accomplished through investigator-initiated projects and unsolicited Letters of Intent (LOIs) from investigators both inside and outside of the clinical trials networks.
Project Teams include extramural investigators, each of whom may fill one or more of the following roles:
- Clinician scientists will be expected to lead the clinical trials that are recommended by the NCI Drug Development Project Team. These trials will be conducted under any or all of the NCI CTEP clinical trials networks. Clinician scientists will be responsible for creating protocol study committees to execute these studies. Junior investigators and their mentors are encouraged to submit Career Development PTMAs (CrD PTMAs), similar to the Career Development Letter of Intent (CrDL) process.
- Translational scientists are expected to provide guidance on prioritization of biomarkers for the studies under development, including recommendations for technologies and platforms that will meet increasingly stringent requirements for integral and integrated biomarkers.
- Basic scientists will be expected to provide scientific guidance for the study design based on the understanding of the mechanism of action of the investigational agent, and to help prioritize the clinical study choices based on published literature and unpublished data. Basic scientists selected to the team will have access to CTEP agents for laboratory studies.
An NCI Drug Development Project Team will meet regularly for 6-8 weeks. At the end of these meetings, the drug development plan will be finalized for presentation to the Investigational Drug Steering Committee (IDSC). Upon approval of the plan by the NCI, the clinician and translational scientists will submit LOIs based on the development plan to CTEP.
After the agent development plan formulated by the project team has been approved by the NCI, and after the agent's collaborative agreement has been signed, CTEP will make the agent available for all qualified investigators (both inside and outside of the clinical trials networks) to submit unsolicited LOIs to conduct clinical trials, or to submit requests to use the agent in nonclinical studies.
A more detailed overview of the NCI’s team-driven drug-development initiative can be found in the ETCTN Guidelines.