CTEP Branches and Offices
Pediatric Early Phase Clinical Trials Network (PEP-CTN)
- Overview/Objectives
- Structure
- PEP-CTN Institutions
- PEP-CTN Agent Prioritization Committee (APC)
- PEP-CTN Resources
- Contact Us
Overview/Objectives
The Pediatric Early Phase Clinical Trials Network (PEP-CTN) strives to enhance NCI's program for conducting early phase clinical trials in children with cancer, building upon the success of the Children's Oncology Group (COG) Phase 1 & Pilot Consortium. The overarching goal is to identify and develop effective new agents for children and adolescents with cancer, through rational and efficient clinical and laboratory research.
The PEP-CTN designs and conducts pediatric early phase trials including phase 1 trials that often include phase 2 expansion cohorts. In addition, the PEP-CTN conducts pilot studies of novel agents/regimens to determine their tolerability so that promising agents/regimens can proceed to definitive testing in phase 3 clinical trials.
Important characteristics of the PEP-CTN include:
- Recognition of the need for seamless transitions from phase 1 to phase 2 testing, reflected by the PEP-CTN name, emphasizing "early phase" clinical trials;
- Establishment of the Pediatric Early Phase Agent Prioritization Committee (APC) to prioritize agents for evaluation by the PEP-CTN and to expedite the pace at which novel investigational agents enter clinical testing in children with cancer;
- Addition of central monitoring for all PEP-CTN clinical trials; and
- Incorporation of relevant biological/genomic evaluations to establish eligibility for PEP-CTN clinical trials and/or to facilitate factors determining the activity of agents studied by the PEP-CTN.
Structure
Dr. Elizabeth Fox (St. Jude Children's Research Hospital) and Dr. Jennifer Foster (Baylor College of Medicine) are the Chair and Vice-Chair, respectively, of the PEP-CTN. Dr. Brenda Weigel (University of Minnesota) is the Immediate Past Chair. Dr. Fox and Dr. Foster also serve as Chair, Vice-Chair, for the Children's Oncology Group (COG) Developmental Therapeutics Committee. The PEP-CTN Operations and Data/Statistics Center (ODSC) is embedded within the COG Operations and Data/ Statistics center. The PEP-CTN is supported by a cooperative agreement award from NCI (UM1-CA228823).
PEP-CTN Institutions
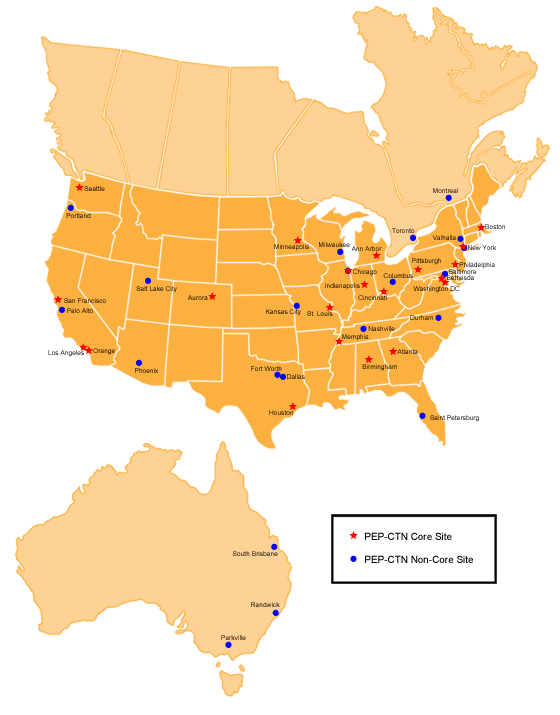
The Pediatric Early Phase-Clinical Trial Network (PEP-CTN) is comprised of 42 premier Children Oncology Group (COG) pediatric sites in the U.S., Canada and Australia. Twenty-one sites are designated as core sites located within the U.S. and participate in all PEP-CTN trials, and 21 non-core member sites in the U.S., Canada and Australia participate in selected trials. All sites were selected through a peer review process. The PEP-CTN serves as a national and international model for new agent development in pediatric oncology. This dedicated consortium provides the necessary infrastructure for the conduct of pediatric early phase clinical trials and pharmacokinetic and biomarker studies.
PEP-CTN Agent Prioritization Committee
The PEP-CTN Agent Prioritization Committee (APC) provides timely, rigorous, and transparent prioritization of investigational agents for evaluation in the childhood cancer setting through the PEP-CTN. The APC is led by the PEP-CTN Chairperson, and its membership includes a range of pediatric drug development stakeholders. Requests for evaluation of agents for testing through the PEP-CTN may be submitted to the APC from PEP-CTN members, COG Disease Committees, other academic research teams, and pharmaceutical companies.
Agents prioritized by the PEP-CTN APC have letters of intent and protocols rapidly developed by the PEP-CTN that are then reviewed by CTEP to ensure that all safety and regulatory issues have been addressed. The process is expedited by the use of protocol templates developed by the PEP-CTN and pre-approved by CTEP for phase 1, phase 1-2, and pilot studies.
The PEP-CTN APC, by providing a single pathway for agents to enter the PEP-CTN, can accelerate the timeline for agents to enter pediatric testing. The APC serves as a single portal by which pharmaceutical companies, PEP-CTN members, COG institutions, or investigators from academia can propose agents/regimens for pediatric early phase clinical evaluation in collaboration with NCI and NCI-supported research teams.
The PEP-CTN APC can also provide a "Pre-Review" of an agent to address questions that an agent's sponsor may have about pediatric development of the agent and about how the PEP-CTN may be able to contribute to the agent's clinical evaluation in children. These discussions are designed for the APC to provide its perspective on specific questions about pediatric development of an agent that are posed by the agent's sponsor. Following a "Pre-Review" meeting, there is no obligation for a company to pursue a collaboration with the PEP-CTN, though should a company wish to do so based on the feedback received, they can proceed with an Agent Prioritization Proposal for their agent.
Those wishing to either request the prioritization of an agent for PEP-CTN evaluation or to request feedback on their agent from the APC should complete an PEP-CTN Agent Prioritization (AP) Proposal form or request via email a "Pre-Review" meeting, respectively. General inquiries and questions about the PEP-CTN AP Proposal form, its submission process, or Pre-Review Meetings can be addressed to: NCI-PEP-CTN@nih.gov. Consultation prior to submission of an AP Proposal form is encouraged.
PEP-CTN Resources
Contact Us
General Inquiries: NCI-PEP-CTN@nih.gov