CTEP Branches and Offices
Radiopharmaceutical Development Initiative (RDI)
Overview/Objectives
As part of NCI’s overall drug development efforts in collaboration with the pharmaceutical industry and academic investigators, NCI has developed a specialized infrastructure, the Radiopharmaceutical Development Initiative (RDI), for the clinical evaluation of novel theranostic radiopharmaceutical cancer therapies. The focus of NCI’s efforts is to complement industry development of these agents with early phase combination studies to test tolerability and early signs of efficacy of radiopharmaceutical agents in combination with other novel agents that may add to their clinical benefit.
The objectives of the RDI are to:
- Conduct early or late phase clinical trials in NCI-funded clinical networks, especially in the Experimental Therapeutics Clinical Trials Network, of NCI-IND radiopharmaceutical agents in combination with other agents in high priority areas of unmet medical needs
- Study dosimetry and radiobiology findings through novel clinical biomarker analyses
- Integrate molecular characterization, pharmacology, cancer biology, and theranostic or dosimetry imaging into radiopharmaceutical-based clinical trials.
See links above for more information on the RDI and the Experimental Therapeutics Clinical Trials Network (ETCTN) or National Clinical Trials Network (NCTN).
RDI Infrastructure
RDI radiopharmaceutical-based trials are supported by integrated centralized clinical and administrative services sponsored by NCI according to the diagram below. For early phase clinical trials of NCI-IND radiopharmaceutical agents, more details and links to services, may be found here.
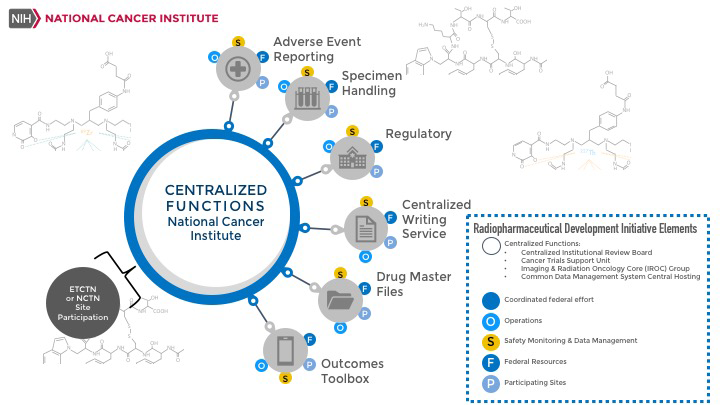
RDI Trials
RDI radiopharmaceutical-based trials involve both early phase and late phase clinical studies of cancer treatments in high priority areas of unmet medical needs. The table below lists active clinical trials for specific diseases or treatment areas.
| CTEP Trial ID | Title | Network | Disease(s) | clinicaltrials.gov website |
|---|---|---|---|---|
| 10096 | Olaparib and Radium-223 Dichloride in Treating Men with Metastatic Castration-Resistant Prostate Cancer that has Spread to the Bone | ETCTN | Prostate | https://clinicaltrials.gov/ct2/show/NCT03317392 |
| 10301 | Radiation Medication (Radium-223 Dichloride) Versus Radium-223 Dichloride Plus Radiation Enhancing Medication (M3814) Versus Radium-223 Dichloride Plus M3814 Plus Avelumab (a Type of Immunotherapy) for Advanced Prostate Cancer Not Responsive to Hormonal Therapy | ETCTN | Prostate | https://clinicaltrials.gov/ct2/show/NCT04071236 |
| 10302 | Testing the Addition of Radium Therapy (Radium-223 Dichloride) to the Usual Chemotherapy Treatment (Paclitaxel) for Advanced Breast Cancer Spread to the Bones | ETCTN | Breast | https://clinicaltrials.gov/ct2/show/NCT04090398 |
| 10388 | Testing the Addition of an Anti-cancer Drug, Triapine, to the Usual Radiation-Based Treatment (Lutetium Lu 177 Dotatate) for Neuroendocrine Tumors | ETCTN | Neuroendocrine | https://clinicaltrials.gov/ct2/show/NCT04234568 |
| A031801 | Testing the Addition of a New Anti-cancer Drug, Radium-223 Dichloride, to the Usual Treatment (Cabozantinib) for Advanced Renal Cell Cancer That Has Spread to the Bone (RadiCal) | NCTN | Kidney | https://clinicaltrials.gov/ct2/show/NCT04071223 |
| 10437 | Treatment of Cancer-Related Bone Pain by Using Bone-Targeted Radiation-Based Therapy (Sn-117m-DTPA) in Patients With Prostate Cancer That Has Spread to Bones | ETCTN | Prostate | https://clinicaltrials,gov/ct2/show/NCT04616547 |
| 10450 | Testing the Addition of An Anti-cancer Drug, M3814 (Peposertib), to the Usual Radiation-Based Treatment (Lutetium Lu 177 Dotatate) for Neuroendocrine Tumors | ETCTN | Neuroendocrine | https://clinicaltrials.gov/ct2/show/NCT04750954 |
RDI White Papers
NCI RDI initiatives consider radiopharmaceutical cancer treatment as any radionuclide administered by vein, injection, inhalation, or ingestion that is intended to irradiate targeted cancer cells while minimizing radiation dose to nearby normal cells. By considering these agents to be drugs from the outset, NCI contends that radiopharmaceuticals follow an easier programmatic path for development to address unmet medical needs. The NCI has published a series of white papers outlining its position on several key trial issues. More details can be found by clicking on the white paper links below.
National Cancer Institute Programmatic Collaboration for Investigational Radiopharmaceuticals
Kunos CA, Capala J.
Am Soc Clin Oncol Educ Book. 2018 May 23;38:488-494. doi: 10.1200/EDBK_200199. Review.
PMID: 30231365
In-Brief: Provides overarching goals of the RDI and its infrastructure needs.
Radiopharmaceuticals for Relapsed or Refractory Leukemias
Kunos CA, Capala J, Ivy SP.
Front Oncol. 2019 Feb 25;9:97. doi: 10.3389/fonc.2019.00097. eCollection 2019.
PMID: 30859091
In-Brief: Provides radiopharmaceutical-related biospecimen handling instructions.
Radiopharmaceuticals for Relapsed or Refractory Ovarian Cancers
Kunos CA, Capala J, Finnigan S, Smith GL, Ivy SP.
Front Oncol. 2019 Mar 26;9:180. doi: 10.3389/fonc.2019.00180. eCollection 2019.
PMID: 30984615
In-Brief: Provides radiopharmaceutical-related pharmacovigilance
Radiopharmaceuticals for Persistent or Recurrent Uterine Cervix Cancer
Kunos CA, Capala J, Kohn EC, Ivy SP.
Front Oncol. 2019 Jun 26;9:560. doi: 10.3389/fonc.2019.00560. eCollection 2019.
PMID: 31297338
In-Brief: Provides radiopharmaceutical-related agent ordering and prescription verification.
Leveraging National Cancer Institute Programmatic Collaboration for Single Radiopharmaceutical Drug Master Files
Kunos CA, Capala J, Ivy SP.
Front Oncol. 2019 Jun 28;9:573. doi: 10.3389/fonc.2019.00573. eCollection 2019.
PMID: 31316916
In-Brief: Provides concepts around single drug master files for radiopharmaceutical new molecular entities.
Clinical Outcome Assessments Toolbox for Radiopharmaceuticals
Kunos CA, Capala J, Dicker AP, Movsas B, Ivy SP, Minasian LM.
Front Oncol. 2019 Oct 10;9:1028. doi: 10.3389/fonc.2019.01028. eCollection 2019.
PMID: 31649885
In-Brief: Provides tools for radiopharmaceutical-related clinical outcome assessments.
Phase 0 Radiopharmaceutical-Agent Clinical Development
Kunos CA, Rubinstein LV, Capala J, McDonald MA.
Front Oncol. 2020 Aug 18;10:1310. doi: 10.3389/fonc.2020.01310.
PMID: 33014772
In-Brief: Provides concepts around phase 0 radiopharmaceutical-agent clinical development.
Radiopharmaceutical Validation for Clinical Use
Kunos CA, Howells R, Chauhan A, Myint ZW, Bernard ME, El Khouli R, Capala J.
Front Oncol. 2021 Mar 3;11:630827. doi: 10.3389/fonc.2021.630827.
PMID: 33747951
In-Brief: Provides steps in the validation of radiopharmaceutical agents for clinical development.
Additional Resources
NCI-sponsored Infrastructure for ETCTN Trials
ETCTN early phase trials are supported by integrated centralized clinical and administrative services sponsored by NCI.
NCI-sponsored Infrastructure for NCTN Trials
National Clinical Trials Network (NCTN) late phase trials are supported by integrated centralized clinical and administrative services sponsored by NCI.
NCI Factoids for Radiopharmaceutical Clinical Trials
Beginning in 2020, early or late phase clinical trials are supported by centralized radiopharmaceutical clinical and administrative services sponsored by NCI. More details can be found by clicking on the factoid link above.





