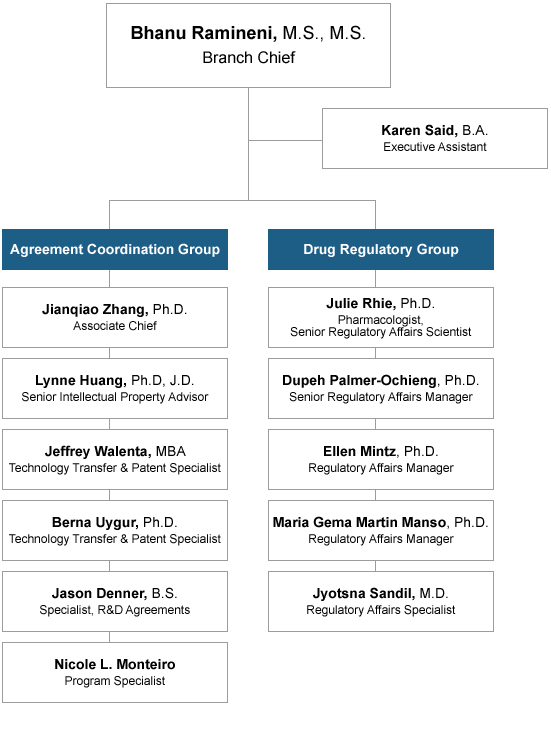
CTEP Branches and Offices
About the Branch Chief
 Bhanu Ramineni, M.S., M.S., became the Chief of the Regulatory Affairs Branch in October 2019. She joined the Drug Regulatory Group in the branch in 2010. She holds two Master's degrees: one in Environmental Sciences from Nagarjuna University, India, and a second one in Biochemistry and Molecular Biology with a specialization in Biotechnology from Georgetown University. Ms. Ramineni comes with significant experience in FDA Regulatory Affairs, as well as preclinical and clinical development of vaccines with a special focus in immunology.
More…
Bhanu Ramineni, M.S., M.S., became the Chief of the Regulatory Affairs Branch in October 2019. She joined the Drug Regulatory Group in the branch in 2010. She holds two Master's degrees: one in Environmental Sciences from Nagarjuna University, India, and a second one in Biochemistry and Molecular Biology with a specialization in Biotechnology from Georgetown University. Ms. Ramineni comes with significant experience in FDA Regulatory Affairs, as well as preclinical and clinical development of vaccines with a special focus in immunology.
More…