Toolkit
CTEP Branches and Offices
Last Updated: 05/30/18
NCI ETCTN Program Infrastructure
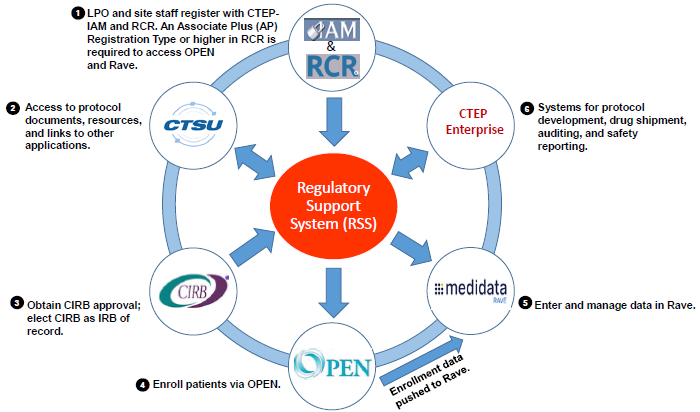
- CTEP-Identity and Access Management (CTEP-IAM)
- Investigators and Associates register for an account that enables access to the other applications (CTSU, OPEN/IWRS, Rave, CTEP Enterprise).
- Cancer Trials Support Unit (CTSU)
- Provides a variety of services, including institution and person roster support; website support for posting of protocols and other information; and links to other services (OPEN/IWRS, Rave).
- NCI Central Institutional Review Board (CIRB)
- Conducts IRB review of most early-phase NCI-sponsored trials, including ETCTN studies.
- Oncology Patient Enrollment Network (OPEN)/Interactive Web Response System (IWRS)
- Linked applications for patient enrollment (OPEN) and slot reservations and cohort management (IWRS). Data will be automatically transferred to Rave.
- Medidata Rave
- An application for data entry, data analysis, and clinical trial management.
- CTEP Enterprise System
- An application for integrated clinical trials management and reporting, including Serious Adverse Event (SAE) reporting through the CTEP-Adverse Event Reporting System (CTEP-AERS); ordering of investigational agents; trial monitoring/audits; and Operational Efficiency Working Group (OEWG) reporting.
- Regulatory Support Services (RSS)
- Serves as a centralized repository for the regulatory documents for all NCI-supported multi-center clinical trials. The RSS provides a streamlined and comprehensive approach to collecting and maintaining site registration, person, and institution documentation essential to the management of clinical trials.





